 November 3, 2022
November 3, 2022 Recalls of joint replacement devices can cause great concern and alarm for both patients and their surgeons, raising questions about whether their health is compromised or if they will require additional surgery.
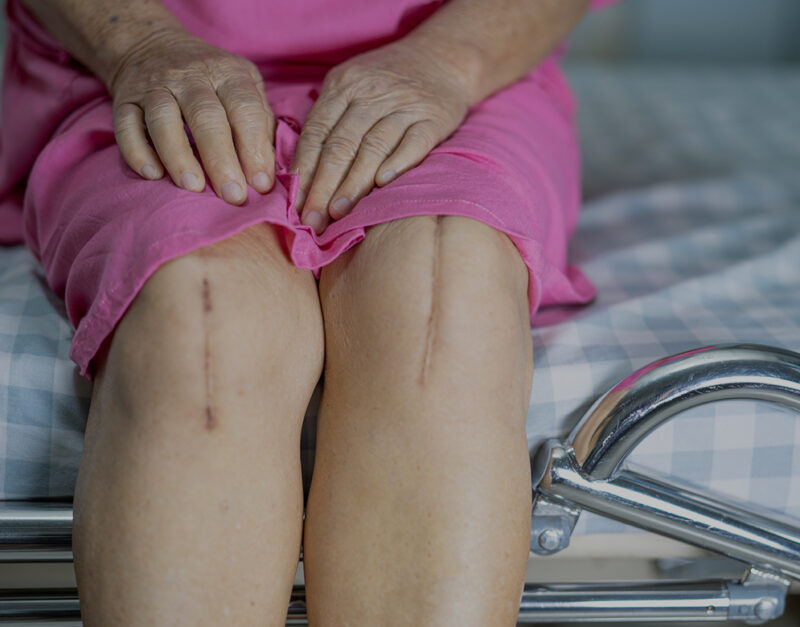
Medical device company Exactech, for example, announced a major recall of all its knee and ankle replacement implant systems made after 2004, prompted by defective packaging that caused a component made from polyethylene to degrade, resulting in many implants to prematurely fail. This has forced many patients to undergo corrective surgeries. The company estimated that more than 143,000 devices are affected.
Here are five questions and answers you should know about joint replacement recalls:
- How do defective joint replacement devices harm patients?
When an implant breaks down, it can cause the device to loosen or fail, leading to potentially severe physical symptoms and mental anguish. Physical health issues from defective joint replacement products may include:
- Damage to the bone, muscle, and nerves near the device
- Infection
- Device loosening
- Clicking, grinding, popping, or other noises
- Dislocations
- Inability to bear weight
- Instability in the knee, ankle, or hip
- New, persistent and/or severe pain
- Bone degeneration
- Premature wear or device failure
- Swelling
- What is the most common joint replacement surgery?
Hip and knee replacement are the most common types of joint replacement surgery. Surgeons perform more than 600,000 knee replacements every year in the United States. Even if only a small percentage of implants fail, flawed devices can injure hundreds or thousands of patients.
Knee replacement lawsuits also are increasingly common due to the rise in joint replacement surgeries as our population ages and technology makes surgeries easier. Claims focus on manufacturing or design flaws that caused them to fail. They cite serious problems that require additional surgery to repair or replace faulty knee implants.
- How do I determine if I am affected by a recall?
The manufacturer of the recalled device and/or your surgeon should send you a letter outlining steps you need to take to address the recall. But patients should take action before waiting for a letter. They may not know the details about their implants, so the first step is to reach out to your health care provider to request records. These medical records should contain the patient’s implant details, including the serial number of the device, which can be checked on the manufacturer’s website.
- What are revision surgeries?
Revision surgeries are additional surgeries doctors perform to correct joint replacement problems. Health care providers typically recommend these when an implant fails or is causing symptoms such as pain, decreased mobility, bone and tissue degeneration or infection.
Failure may be caused by a defective implant or with time. Most implants last between 15 to 20 years in 85% to 90% of patients, according to Hospital for Special Surgery.
Revision surgeries are more difficult to perform than primary joint replacement procedures and they require planning and special implants. They take longer to perform and may have more complication risks. Some patients require more than one revision surgery.
- What are my legal options?
Patients affected by recalled joint replacements may have several compensation options, including:
Product liability lawsuit: This form of personal injury lawsuit holds companies responsible for injuries caused by defective products. Defective medical devices commonly lead to this type of lawsuit. If a verdict favors the injured patient, it may award them money as compensation for injuries.
Settlement: A settlement might be negotiated by the patient’s lawyer without going to trial. This can save time and money, but often at a price. A settlement may award the patient less money than a lawsuit. It may also require the patient to give up the right to file a lawsuit.
Patients may want to consult a lawyer first before taking any action.
How We Help Medical Device Victims
Seek justice with the help of our experienced attorneys. Our Dallas, Texas, medical device law firm has battled corporate giants on behalf of individuals like you for 20 years, aggressively fighting to hold them responsible for dangerous products. If you or a loved one suffered catastrophic injury or death caused by unsafe medical devices, we can help.


