 March 22, 2016
March 22, 2016 Metal-on-metal hip replacement manufacturers must now submit a premarket approval application (PMA) to the U.S. Food and Drug Administration for two types of hip systems, one having a cemented acetabular component and the other having an uncemented acetabular component. The FDA’s final order released this past February reportedly gives medical device makers until May 18 to submit their new applications.
FDA Orders Metal-On-Metal Hip Implant Makers to Obtain Premarket Approval Prior to Selling Certain Devices
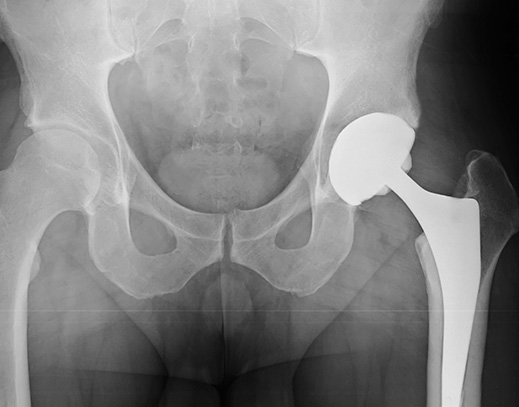
For years, U.S. doctors and patients have reported problems with metal-on-metal hip replacements to the U.S. Food and Drug Administration (FDA). Describing the evidence as “insufficient” to ensure the safety of metal-on-metal hip systems, the FDA has decided to strengthen its regulation of the devices.
Since 2013, the FDA has warned that metal-on-metal hips pose “unique risks.” The metal cup and ball can rub against each other, causing pain and releasing metal particles into the surrounding bone and soft tissue. Continuing erosion of the hip implant may eventually cause the device to fail. In many cases, patients have been required to undergo painful revision surgery to remove the defective metal-on-metal hip implant and replace it with a different system.
The FDA’s PMA application will require device makers to disclose “any risks known, or that should be reasonably known” about their metal-on-metal hip replacements as well as “full reports of all nonclinical and clinical information from investigations on the safety and effectiveness of the device.”
Contact Waters Kraus Paul & Siegel to Learn More About Filing a Metal-on-Metal Hip Implant Lawsuit
Waters Kraus Paul & Siegel is a national plaintiffs’ law firm devoted to helping families in personal injury and wrongful death cases involving dangerous medical devices manufactured by a number of companies, including Stryker, Zimmer, Biomet and DePuy. If you have suffered injuries or the death of a loved one associated with metal-on-metal hip replacements, contact us by email or call us at 800.226.9880 to speak with one of our medical device attorneys, like Sara Coopwood in the firm’s Texas office, and learn more about how we can assist you with a metal-on-metal hip replacement lawsuit or claim.


